THERMOREVERSIBLE CROSS-LINKING
Melt processing techniques (such as injection molding and extrusion) are applied readily to thermoplastic polymers, and are in fact the most commonly used thermoplastic polymer processing techniques. By contrast, melt processing techniques cannot be applied to thermoset polymers, since thermoset polymers comprise a three-dimensional network of covalent bonds so that they are unable to melt and flow.
The inability to apply melt processing techniques limits the processing options available for thermoset polymers greatly as compared with the processing options available for thermoplastic polymers. For example, thermoset articles cannot be manufactured by subjecting a melt of the polymer to injection molding or to extrusion. It is also impossible to use simple mechanical recycling technologies for post-consumer thermoset polymer products and to thermoset factory scraps since mechanical recycling includes a melt processing (usually extrusion) step that cannot be performed for a thermoset polymer.
Such processing limitations of thermoset polymers have led to a significant amount of work on the development of polymers with thermoreversible cross-linking. The cross-links in such a polymer are broken when processing conditions are applied, while they re-form so that the polymer behaves as a thermoset during use. For example, the cross-links of a thermoreversibly cross-linked polymer break down when the polymer is heated up, so that it becomes thermoplastic and it can be fabricated into articles by using standard melt processes. The cross-links re-form when the fabricated articles are cooled down so that the fabricated articles provide the advantages of being thermosets under the use conditions. Post-consumer fabricated articles, as well as factory scraps, are subjected to mechanical recycling by heating up to reverse the cross-linking again and to thus allow the melt processing (usually extrusion) step of mechanical recycling to be applied.
Many different reaction chemistries have been tested for use to create thermoreversible cross-linking, some with considerable success. The following are the two classes of techniques that have been used most commonly for this purpose:
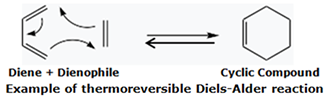
- Use of conjugated diene cross-linkers to perform the Diels-Alder reaction with a dienophile. The Diels-Alder reaction is thermoreversible. Hence a suitable conjugated diene can often be selected to build a molecular architecture where the polymer possesses a covalently cross-linked network at low temperatures, becomes capable of thermoplastic behavior as a result of the thermoreversion of the Diels-Alder reaction upon heating, and becomes a covalently cross-linked network again when a fabricated article is cooled. The versatility of the Diels-Alder reaction has enabled its application, at least at the laboratory scale, to the development of thermoreversibly cross-linked versions of many polymers, including many commonly used rubbers and elastomers.
![Example of Thermoreversible Diels-Alder Reaction Example of Thermoreversible Diels-Alder Reaction]()
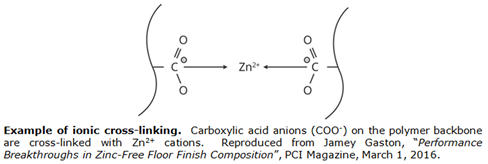
- Use of ionic cross-links formed by association of cationic and anionic structural units present simultaneously (often as pendant groups) on polymer chains instead of covalent cross-links. Most commonly, anionic entities are attached to the polymer chains while the cations are metallic or organic or organometallic ions. However, systems where cationic entities are attached to the polymer chains, and systems where both anionic and cationic entities are attached to the polymer chains, also exist. Ionic cross-links tend to be less stable than covalent cross-links so that an ionically cross-linked polymer may behave like a thermoset polymer at low temperatures, may become capable of thermoplastic behavior as a result of the breakage of the ionic cross-links upon heating, and may behave like a cross-linked polymer again when a fabricated article is cooled.
![Example of Ionic Cross-linking]()
Among the techniques that have been used much less frequently, the creation of supramolecular hydrogen bonding networks may be the most noteworthy.
It is interesting to note that, regardless of the class of technique used (Diels-Alder reaction, ionic cross-linking, or supramolecular hydrogen bonding), maleic anhydride is the most commonly used reagent in the creation of thermoreversibly cross-linked polymers.